Cyclobutadiene Smaller on:
[Wikipedia]
[Google]
[Amazon]
Cyclobutadiene is an
 The Dewar benzene converts to
The Dewar benzene converts to 
organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The ...
with the formula . It is very reactive owing to its tendency to dimerize
A dimer () ('' di-'', "two" + ''-mer'', "parts") is an oligomer consisting of two monomers joined by bonds that can be either strong or weak, covalent or intermolecular. Dimers also have significant implications in polymer chemistry, inorganic ch ...
. Although the parent compound has not been isolated, some substituted derivatives are robust and a single molecule of cyclobutadiene is quite stable. Since the compound degrades by a bimolecular
In chemistry, molecularity is the number of molecules that come together to react in an elementary (single-step) reactionAtkins, P.; de Paula, J. Physical Chemistry. Oxford University Press, 2014 and is equal to the sum of stoichiometric coeffici ...
process, the species can be observed by matrix isolation
Matrix isolation is an experimental technique used in chemistry and physics. It generally involves a material being trapped within an unreactive matrix. A ''host'' matrix is a continuous solid phase in which ''guest'' particles (atoms, molecules, i ...
techniques at temperatures below 35 K. It is thought to adopt a rectangular structure.
Structure and reactivity
The compound is the prototypical antiaromatic hydrocarbon with 4 π-electrons. It is the smallest 'n''annulene
Annulenes are monocyclic hydrocarbons that contain the maximum number of non-cumulated or conjugated double bonds (' mancude'). They have the general formula CnHn (when ''n'' is an even number) or C''n''H''n''+1 (when ''n'' is an odd number). The ...
( annulene). Its rectangular structure is the result of the Jahn–Teller effect
The Jahn–Teller effect (JT effect or JTE) is an important mechanism of spontaneous symmetry breaking in molecular and solid-state systems which has far-reaching consequences in different fields, and is responsible for a variety of phenomena in sp ...
, which distorts the molecule and lowers its symmetry, converting the triplet to a singlet ground state. The electronic states of cyclobutadiene have been explored with a variety of computational methods. The rectangular structure is consistent with the existence of two different 1,2-dideutero-1,3-cyclobutadiene valence isomer In organic chemistry, two molecules are valence isomers when they are constitutional isomers that can interconvert through pericyclic reactions.
Benzene
There are many valence isomers one can draw for the C6H6 formula benzene. Some were originally ...
s. This distortion indicates that the pi electron
In chemistry, pi bonds (π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbitals ...
s are localized, in agreement with Hückel's rule
In organic chemistry, Hückel's rule predicts that a planar ring molecule will have aromatic properties if it has 4''n'' + 2 π electrons, where ''n'' is a non-negative integer. The quantum mechanical basis for its formulation was fir ...
which predicts that a π-system of 4 electrons is not aromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
.
In principle, another situation is possible. Namely, cyclobutadiene could assume an undistorted square geometry, ''if it'' ''adopts a triplet spin state''. While a theoretical possibility, the triplet form of the parent cyclobutadiene and its substituted derivatives remained elusive for decades. However, in 2017, the square triplet excited state of 1,2,3,4-tetrakis(trimethylsilyl)-1,3-cyclobutadiene was observed spectroscopically, and a singlet-triplet gap of ''E''ST = 13.9 kcal/mol (or 0.6 eV per molecule) was measured for this compound.
Synthesis
Several cyclobutadiene derivatives have been isolated with steric bulky substituents. Orange tetrakis ( ''tert''-butyl)cyclobutadiene arises bythermolysis
Thermal decomposition, or thermolysis, is a chemical decomposition caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes. The reaction is usually endothermic as heat is req ...
of its isomer tetra-''tert''-butyltetrahedrane
Tetrahedrane is a hypothetical platonic hydrocarbon with chemical formula and a tetrahedral structure. The molecule would be subject to considerable angle strain and has not been synthesized as of 2021. However, a number of derivatives have be ...
. Although the cyclobutadiene derivative is stable (with respect to dimerization), it decomposes upon contact with .
Trapping
Samples of cyclobutadiene are unstable since the compounddimer
Dimer may refer to:
* Dimer (chemistry), a chemical structure formed from two similar sub-units
** Protein dimer, a protein quaternary structure
** d-dimer
* Dimer model, an item in statistical mechanics, based on ''domino tiling''
* Julius Dimer ...
izes at temperatures above 35 K by a Diels-Alder reaction. By suppressing bimolecular decomposition pathways, cyclobutadiene is well-behaved. Thus it has been generated in a hemicarceplex. The inclusion compound
In host–guest chemistry, an inclusion compound (also known as an inclusion complex) is a chemical complex in which one chemical compound (the "host") has a cavity into which a "guest" compound can be accommodated. The interaction between the ho ...
is generated by photodecarboxylation of bicyclopyran-2-one. When released from the host–guest complex, cyclobutadiene dimerizes and then converts to cyclooctatetraene
1,3,5,7-Cyclooctatetraene (COT) is an unsaturated derivative of cyclooctane, with the formula C8H8. It is also known as nnulene. This polyunsaturated hydrocarbon is a colorless to light yellow flammable liquid at room temperature. Because of ...
.
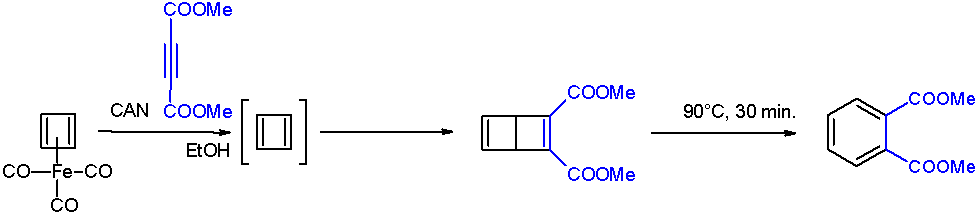
After numerous attempts, cyclobutadiene was first generated by oxidative degradation of cyclobutadieneiron tricarbonyl
Cyclobutadieneiron tricarbonyl is an organoiron compound with the formula Fe(C4H4)(CO)3. It is a yellow solid that is soluble in organic solvents. It has been used in organic chemistry as a precursor for cyclobutadiene, which is an elusive specie ...
with ammonium cerium(IV) nitrate
Ceric ammonium nitrate (CAN) is the inorganic compound with the formula . This orange-red, water-soluble cerium salt is a specialised oxidizing agent in organic synthesis and a standard oxidant in quantitative analysis.
Preparation, properties ...
. When liberated from the iron complex, cyclobutadiene reacts with electron-deficient alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
s to form a Dewar benzene
Dewar benzene (also spelled ''dewarbenzene'') or bicyclo .2.0exa-2,5-diene is a bicyclic isomer of benzene with the molecular formula C6H6. The compound is named after James Dewar who included this structure in a list of possible C6H6 structures in ...
:
: The Dewar benzene converts to
The Dewar benzene converts to dimethyl phthalate
Dimethyl phthalate is an organic compound and phthalate ester. it is a colourless and oily liquid that is soluble in organic solvents, but which is only poorly soluble in water (~4 g/L).
It is used in a variety of products and is most commonly us ...
on heating at 90 °C.
One cyclobutadiene derivative is also accessible through a +2 ycloaddition of a di-alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
. In this particular reaction the trapping reagent In chemistry, a chemical trap is a chemical compound that is used to detect unstable compounds. The method relies on efficiency of bimolecular reactions with reagents to produce a more easily characterize trapped product. In some cases, the trappin ...
is ''2,3,4,5-tetraphenylcyclopenta-2,4-dienone'' and one of the final products (after expulsion of carbon monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simple ...
) is a cyclooctatetraene
1,3,5,7-Cyclooctatetraene (COT) is an unsaturated derivative of cyclooctane, with the formula C8H8. It is also known as nnulene. This polyunsaturated hydrocarbon is a colorless to light yellow flammable liquid at room temperature. Because of ...
:
:
See also
*Butadiene
1,3-Butadiene () is the organic compound with the formula (CH2=CH)2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two viny ...
*Cyclobutene
Cyclobutene is a cycloalkene. It is of interest in research but currently has no practical applications. It is a colorless easily condensed gas. A modern synthesis involves the 2-step dehydration of cyclobutanol. The compound was first prepared ...
References
{{Annulenes Annulenes Antiaromatic compounds Four-membered rings